The Carbon Removal Primer
A comprehensive overview of carbon dioxide removal (CDR)
You’re reading Terraform Now, a newsletter on the business of carbon removal. To support my work, you can subscribe here or forward this article with interested folks:
Carbon removal is one of the many solutions that can help us tackle the colossal challenge of global warming. The idea behind carbon removal is quite simple: remove greenhouse gases from the atmosphere and store them where they won't escape, reducing the impact of global warming. Carbon dioxide (CO2) is the easiest gas to remove because it is much more common than methane, nitrous oxide, and other gases. This is why all feasible greenhouse gas capture projects focus on carbon dioxide.
Carbon removal is not optional — humanity needs this solution to scale in the coming decades. To learn more about why we need it, check out Terraform Now’s Case for Carbon Removal.
Fully understanding carbon removal requires putting on different hats — we need to think like a chemical engineer, geologist, CEO, and policy analyst. This Substack exists because I can’t find anywhere on internet, in a book, or in the scientific literature that clearly and succinctly lays out what this technology is, why it matters, and how the carbon removal business landscape is likely to evolve.
New companies and ideas are popping up almost daily in this space. There are already many technologies that remove CO2, some of which are more or less efficient, more or less mature, and each with pros and cons. We pull CO2 out of the atmosphere, out of flue gases from power plants, and from water. We use plants to remove CO2, and algae, and chemicals in the ocean.
And we can do many things with CO2. We use it to make biofuels, to pump more oil out of the ground, and in industrial processes. Ultimately, we’ll need bury almost all of the CO2 we capture underground. In this post, you’ll get an overview of each element of carbon removal, with links to deep dives on areas where there is a lot more to say.
Let’s start with what actually happens in carbon removal. There are a few key steps: capturing CO2, preparing it, trasporting it, and either storing or using it:
Let’s look at each of these in turn.
CAPTURING CO2
There are three ways we take CO2 out of the biosphere:
(A) At Emissions’ Source: We capture CO2 and other greenhouse gases where they are created — think about installing a device in the the smokestack of a natural gas plant, or, less plausibly, on the tailpipe of a semi-truck, that takes CO2 out of the exhaust.
Emissions’ Source carbon capture is a technology for pragmatists who want to make an immediate impact on climate change. It’s scaling today and can be relatively cheap, but limited in its long-term potential to reverse climate change.
(B) Direct Air Capture: This means capturing the carbon dioxide ‘out in the wild,’ directly from the environment. Industry experts lump many solutions into “Direct Air Capture” but it’s worth noting that not all the solutions interact directly with the atmosphere. For example, bodies of water naturally extract CO2 out of the atmosphere, and some companies are using this property of water in their DAC solutions.
Direct Air Capture is most expensive and ambitious option. It’s for optimists who believe in the power of incremental technological innovation and experience curves to reduce costs.
(C) Organic Capture: This uses natural processes to store carbon. Because the carbon cycle is relatively complex, there are many ways to organic processes that we can tune or accelerate capture and store CO2, usually in the form of other long-chain carbon molecules. To name a few:
Trees take in CO2 and use the carbon as a core building block of just about every molecule. Planting forests has been an accepted way to fight climate change for decades.
Algae in the ocean can work by a similar process. There are proposals to seed the ocean with large amounts of Iron, which would create large algae blooms. This idea has been largely discredited by sane scientists, since it might wreck marine ecosystems, but that doesn’t mean that it’s not an option for less grounded actors.
When farming by-products decay, they gives off carbon dioxide, methane, and other greenhouse gases. Some firms are pioneering a process where we turn these agricultural by-products into a stable Biochar or Bio-oil
This last example highlights a unique and exciting aspect of Organic Capture: it can act on more than just carbon dioxide. Methane, nitrous oxide, and other greenhouse gases are in play. This will make it a key pillar of the Carbon Capture toolkit, even if the more technical solutions become cheaper.
Organic capture is the least industrial option, and has the greenest vibes — so it’s often the preferred option of environmentalists. It is also the cheapest, requiring little in the way of materials or energy.
Each of these ways to capture CO2 have advantages and drawbacks:
Evaluating solutions on their potential to reverse global warming — the goal of carbon removal — is essential but tricky. There are two bits to keep in mind here:
Speed: How quickly can a solution remove CO2? Capturing at the (a) Emissions’ Source and via (b) Direct Air Capture are both immediate, while some (c) Organic Capture use cases take a long time. While Organic Capture can be accelerated by choosing the right species and perhaps genetically modifying them, trees take decades to sequester the bulk of their carbon. There are some cases where Organic Capture that is faster, like Biochar.
Emission coverage: How much CO2 can a given solution remove? Capturing at the (a) Emissions’ Source has scaling limitations. It can only capture current emissions, and only at sites that are appropriate for carbon capture, which represents around ~50% of current emissions. Meanwhile (c) Organic Capture faces twin problems, a land-use tradeoff and a total land constraint. The trade-off is that land used to plant trees could also be used for farming or nature preserves. And the constraint is that there’s only so much arable land on earth, putting an upper bound on the amount of land that could be used to sequester carbon.
Direct Air Capture is the clear winner on the potential to reverse global warming because:
Unlike Emissions’ Source technology, it has the potential to cover all emissions
Unlike Organic Capture, it can capture historic emissions in years rather than centuries.
The reason DAC is important is because, at some point, we have to reckon with our “debt” of CO2, as historic emissions1 are about ~30x of what is emitted in any given year.
Real success with Emission’s Source requires global adoption. American, Chinese, German, and Indian power plants all have to promote this technology. Then, coal-fired plants, natural gas plants, dumpsites, and other scaled sites of emissions all need to install carbon capture technology, at a significant investment. Even if all of this happens, Emissions’ Source technology can only cover ~50% of in-year emissions.
To put this in context, if the USA installed Emissions’ Source equipment on every single power plant and dumpsite, it would capture 5-6% of global emissions in any given year.
Direct Air Capture (DAC from now on) is different. One large, rich, and motivated country could theoretically scale up DAC and — because that country shares the atmosphere and oceans with other countries — counteract CO2 emissions from all over the world, past and present. To be clear, this is unrealistic today because it is too expensive. But that’s a resource constraint, which could go away if economies continue to grow. Geopolitical constraints are likely here to stay.
NOW FOR THE BAD NEWS
OK, since DAC has so many virtues, why don’t we massively scale it? There’s one obstacle: it’s really expensive. DAC requires more energy, heat materials than Emissions’ Source tech, and this is true for all four established DAC technologies.
CO2 is 0.04% of Earth’s atmosphere and 6-12% for flue gases in natural gas and coal plants. This means DAC plants have to circulate at least 100x more air through its turbines, and that’s exactly what we see in the real-world. Luckily, DAC does not need to be 100x the cost. A number of new, thoughtful designs enable DAC to deliver a cost that is only 20x Emissions’ Source costs.
But $1,000 per ton is still way too expensive! So is $600, or $300! Some companies and academics believe there’s room to bring DAC costs down considerably, through new processes of extracting CO2 from the atmosphere. One example from a firm called Carbon Engineering uses water to pull CO2 out of the atmosphere, then absorbs the CO2 out of the water. This has a few advantages — cheaper materials, less energy spent, etc. We’ll cover this solution in more depth in the forthcoming deep dive on DAC. For now it’s worth noting that while scientists and company leaders have written papers suggesting a cost of ~$100 per ton of CO2, this has not been validated in the real world.
Other ways to lower DAC costs include using otherwise unused energy sources. This is what a firm called Climeworks is doing by building DAC facilities on volcanic sites in Iceland, allowing them to use free-ish geothermal energy.
The long-term direction of DAC costs is up for debate. Well that’s putting it kindly — it’s more of a holy war between pragmatists and optimists. Pragmatists think that getting below $600 per ton given basic energy and material calculations. Optimists point to many processes that have become vastly more efficient as they scale, like solar photovoltaic and battery production. I lean slightly toward the optimists, with very little evidence. There’s so much that needs to go right: material costs must decline, there has to be an abundance of cheap, low-carbon energy, and government support to enable these solutions to get big enough to start seeing economies of scale.
TRANSPORTING CO2
After CO2 has been pulled from the atmosphere or the flue gas of a power plant, it needs to be moved to the site where it will be stored and used. The first thing to note about transporting CO2 is that it must be in liquid form, not gaseous form like it is in the atmosphere. This means that at the end of most capture processes, the CO2 needs to be compressed to turn it into a liquid.
There are three common ways to move CO2 — via pipeline, ship, and truck:
There are two bits of good news on the transport front:
Transporting CO2 is cheap, at least relative to capturing it
Pipelines, ships, and trucks are well-known technologies, operating at scale
Right now, transporting and storing CO2 is not an issue with Organic Capture, since the carbon is stripped from the oxygen and stored in the superstructures living things — usually trees. As Organic Capture use cases multiply, this is likely to change. Eventually we’ll probably use pipelines, ships, and trucks to transport CO2-infused by-products of Organic Capture, like synthetic oils, to their final destinations for use or storage.
USING AND STORING CO2
99%+ of CO2 we capture at Emissions’ Source or via DAC will eventually be stored underground, So carbon capture companies that plan to be around for the long haul will want to get very good at all aspects of underground storage in the coming years.
That said, many companies operating in 2023 are excited about what the industry calls “utilization” of the CO2. There are many existing industrial use cases for CO2, including carbonating soda, producing recycled fuels, and manufacturing fertilizer. While it’s true that CO2 is used in these processes, it’s unlikely to be a great long-term play. There are a few reasons for this:
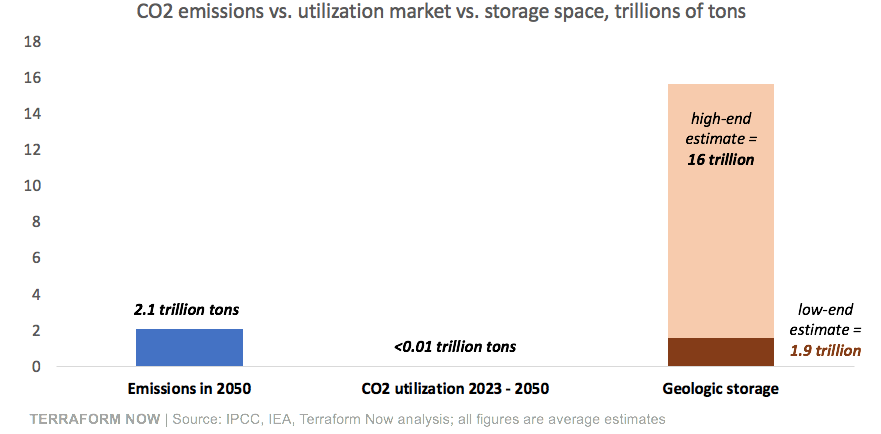
The size of the CO2 utilization market is small, and growing slowly: Right now, ~230 million tons of CO2 are used in industrial processes, compared to the 38 billion tons of CO2 we emit every year. It would take 165 years of industrial CO2 demand to use one year of emissions, and ~5,000 years to use our historic emissions still in the atmosphere (1.1 trillion tons). Compared to the vast geologic space we have to store CO2, the industrial CO2 market is simply tiny:
End-markets for CO2-infused products are not growing fast enough to expand utilization at the rate we’d need to see for it to ‘matter,’ even in the medium-term. Take fertilizers, which are 60% industrial CO2 market. This is a mature market growing at <5% per year.
For the kind of explosive growth we’d want to see for utilization to be a long-term play, we’d have to hope for moonshots — things like massive demand for graphene to make space elevators. This is (literally) outlandish.
Captured CO2 is expensive compared to the existing CO2 supply chain: Companies already produce CO2 at prices of ~$25 per ton of CO2, compared $600+ for DAC and $50+ for capturing at Emissions’ Source. Unless captured CO2 is subsidized by governments, its hard to see how it can displace current suppliers.
CO2 used in most utilization processes is likely to be re-released as CO2 into the atmosphere, negating the time & energy spent capturing it. This is true of fertilizers, soda, and other use cases, but the one we’ll focus on, by way of example, is recycled fuels, also called e-fuels or synthetic fuels. The idea is to use captured CO2 + water to make synthetic gasoline, primarily for cars. A few things are true about these ‘recycled’ fuels:
Recycled fuels cannot reduce emissions, since they will combust, with as much CO2 as was originally captured re-entering the atmosphere as a by-product
Using a recycled fuel in your car is better than using oil that was just pulled out of the ground
In theory, recycled fuels won’t increase emissions, since they are part of a process that pulls CO2 out of the biosphere and then re-uses it
In reality, recycled fuels will almost always increase emissions because the recycling process itself is not free. It requires materials, energy, and money — carbon capture is but one part of that process. Being completely carbon neutral in creating synthetic fuels is not easy. You need renewable energy, which is possible, and renewable materials to capture CO2, which is hard.
Recycled fuels are better than what we’re currently doing, but they not an ideal solution — a bit like transitioning our power plants from coal to natural gas. To be clear, this is not an invidious comparison! In the United States, the transition from coal to natural gas has been the biggest contributor to declining emissions. Carbon capture needs to start somewhere, and recycled fuels is not a bad option.
Before we dive into storage, it’s worth noting one case that straddles the line between “utilization” and “storage” of captured CO2. This is Enhanced Oil Recovery (EOR)2, a nifty process that pumps CO2 into oil wells that are almost dry, pushing the last bits of oil out of the well. A lot of environmentalists hate EOR, in part because they despise anything that makes money for oil & gas companies, and because they don’t want more oil coming out of the ground. But if you assume that the oil was going to be pumped anyway, it’s a nice by-product that EOR stores at least some CO2 underground, making it better at the margin than standard O&G practice. Today, oil companies use EOR to put 80 million tons of CO2 underground each year — almost twice as much as the entire carbon capture industry (45 million tons).
THE LONG-TERM SOLUTION: UNDERGROUND STORAGE
This brings us to storing CO2 underground. Relative to CO2 utilization, storage has two massive advantages. First, there is enough space underground to store all historic and current emissions, many times over. The high-end estimate of geologic storage that can safely store CO2 is ~16 trillion tons. The amount of CO2 humans emitted since 1850*, plus the amount we will emit from 2023 until 2050 is ~2.1 trillion tons — so storage capacity could outpace the CO2 we need to capture by ~8x.
If you want to be pessimistic and believe the low-end storage estimates, it will be a long time before we need to worry about running out of storage. Today we are only removing 45 million tons of CO2 per year, less than 0.001% of the ~1.9 trillion ton low-end estimate.
The second advantage of underground storage is that, when done correctly, it can permanently remove CO2 from the atmosphere. No combustion, no leaks — pure negative emissions. So, what does it mean to store CO2 correctly? Glad you asked:
The ground has to be porous and permeable to allow CO2 to find pockets where it can settle
The cavern must have a hard caprock, a large dome of solid rock, to keep CO2 from leaking out
The cavern must be at least 800 meters deep to create enough pressure to keep CO2 in its liquid form
The cavern should be as big as possible, since larger formations allow us to store more CO2
Fortunately, there are three types of geologic formations that almost always have these qualities: deep aquifers, emptied-out oil and gas wells, and basalt structures created by old lava flows.
Deep aquifers are like small underground oceans, but with more salt and minerals. These are not the same freshwater aquifers that could be used for drinking water. Water in these aquifers naturally absorbs CO2, so all we have to do is pump it about a kilometer underground down a shaft. The newly CO2-infused water is heavy and drifts to the bottom of the aquifer, bringing un-infused water to the top. This un-infused water can then take more CO2, a cycle which repeats until the aquifer is at capacity.
Empty oil and gas wells come in all sorts of different shapes and sizes, but they have one thing in common: for millions of years, they trapped vast amounts of liquid with minimal leakage. As a result, they are almost always good candidates to store liquid CO2. They usually have pre-existing shafts that will just need to be unplugged. While these wells are better storage caverns than deep aquifers, they are a bit more rare, so we’ll likely fill them up first before moving on.
Basalt structures were created by volcanic eruptions and typically make up 90% of any given lava flow. The caprock for these basalt structures is usually made by a more dense lava flow from a later eruption, but the cavern itself is a little different than aquifers or O&G wells. Instead of being one large, continuous cavern, basalts have many tiny, connected holes that absorb CO2. If an aquifer or O&G well is a bucket, then a basalt structure is a sponge. One other interesting aspect of basalts is that the CO2 hardens to become part of the basalt structure — a process known as mineralization.
Storage is not as simple as choosing a good site, dumping the CO2, and letting it be. Keeping it safe and compliant with regulations means monitoring a whole bunch of things:
Pressure of the whole underground system
Potential of the system to cause earthquakes
Location of the CO2 in the geologic formation
Location of salty & mineralized water relative to underground freshwater
Interaction between storage sites
As you can tell there is a lot more going on with storage — including the actual physics of how this works, the dangers of storage, and the geographic distribution of storage. I wrote a multi-part series on storage that starts here.
Historic emissions in this case is actually “historic CO2 emissions since 1850 minus all of the CO2 that has been absorbed by the ocean, long-living trees, and other semi-permanent parts of the natural carbon cycle”
The IEA classified Enhanced Oil Recovery as “utilization” because it serves a purpose of pumping oil out of the ground. But because the end result is storing CO2 underground permanently, it seems more like storage to me. I can see why the utilization moniker stuck, because the process leads to oil extraction, which leads to CO2 combustion. That’s why it straddles the line!
Opinions expressed on Terraform Now do not represent the views of my employer, Bain & Company. Facts presented here are publicly sourced unless otherwise noted.